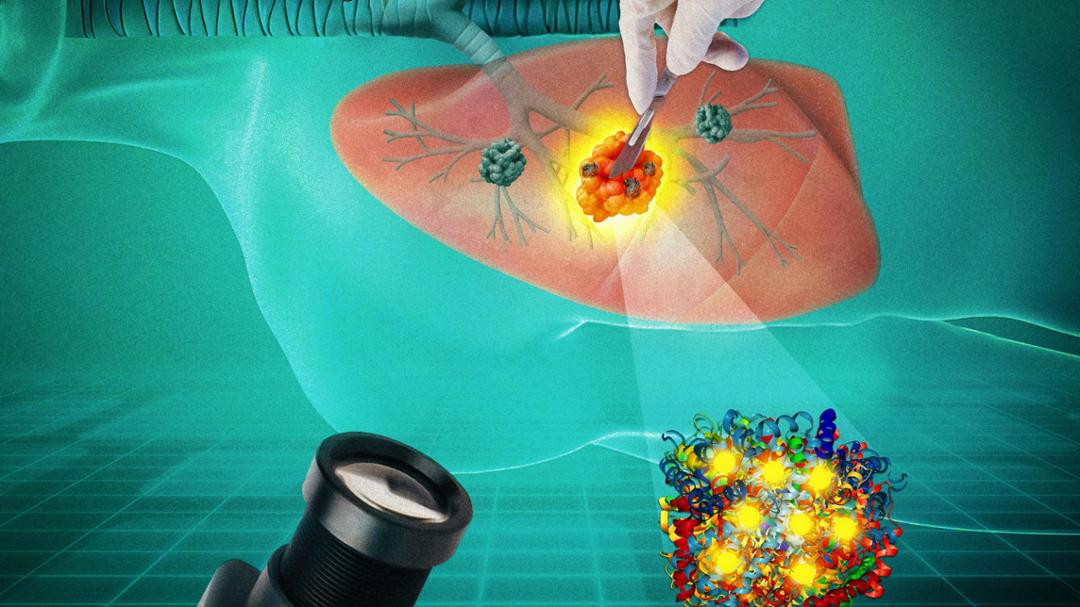
Indrajit Srivastava has discovered how to make light linger in tumors to ensure more accuracy in the removal process.
Afterglow has been on Indrajit Srivastava’s mind nearly this entire year.
But he insists, not the lyrics of the Taylor Swift song. Rather, a way to provide hope to cancer patients and surgeons alike through an improved surgery technique.
“This is challenging the current paradigm of fluorescence-guided surgery,” he said. “We are excited about the potential it has.”
Srivastava brought his expertise in surgical technologies to Texas Tech University in fall 2023 as an assistant professor in the Department of Mechanical Engineering in the Edward E. Whitacre Jr. College of Engineering. It took him about six months to set up his lab. Then, he began to share about afterglow imaging with a couple of undergraduate students who would become lead authors of his research published last month in “Advanced Functional Materials.”
Srivastava explained that fluorescence-guided surgery is the current standard for tumor removal in an operating room, in which dye is injected that turns tumors fluorescent when illuminated with a laser. This enables surgeons to identify tumors from healthy tissue and be more efficient in the removal process.
However, there are limitations. Once the laser is turned off, the fluorescent light disappears – but constantly shining the laser is not an option. This exposure would bleach the dye and destroy its signal.
Fluorescence also can only penetrate a few millimeters, so it is not visible through deep tissue. It even scatters, which makes it hard to differentiate a tumor from surrounding healthy tissues.
“If the tumor is buried deep below an organ, say lungs, you won’t be able to see it,” Srivastava explained. “So, you might miss it during surgery because the fluorescent light could not travel through.”

Srivastava discovered that afterglow imaging, developed in the last few years, faced none of these limitations. After the initial illumination from the laser, an effect he explains is similar to glow-in-the-dark stickers is created as the light persists for up to 10 minutes and allows surgeons time to be more precise.
“It’s recurring, so you can shine the laser, close it and do the surgery,” Srivastava explained. “Then, once you want to check if all the tumor is removed, you can shine the light again. If nothing glows, that means you got it all out. If something glows, then you can use that to do secondary surgeries.”
To advance afterglow imaging one step closer to widespread adoption, Srivastava decided to adopt a strategic repurposing of afterglow materials with proteins to amplify their signals. This enhancement through protein complexation creates a penetration depth of up to one centimeter.
For the past six months, Srivastava and his team comprising of undergraduate and graduate students at The Srivastava Lab worked vigilantly to gather these findings and submit their abstract. The next step Srivastava intends to take is to further explore afterglow imaging agents to make this research more clinically adaptable by applying for federal funding. He also plans to utilize support from Texas Tech’s One Health initiative for funding and acquiring collaborators for grants and future publications.
In the meantime, he looks forward to spreading news of this work at national meetings and conferences – a chance to reflect on his potential impact as a scientist.
“It’s very fulfilling, challenging myself to come up with scientific ideas that can potentially improve current clinical technologies,” he said. “You have to always keep challenging the current technologies because nothing is foolproof. There’s always room for improvement, and I think that’s what we need to do as scientists.”